Mechanism of a cobalt-catalyzed hydroarylation reaction and origin of stereoselectivity†
Abstract
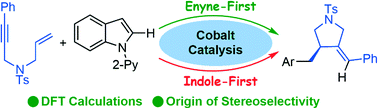
In the present study, the mechanism of a cobalt-catalyzed hydroarylation reaction between N-pyridylindole and 1,6-enynes and the origin of its stereoselectivity have been systematically investigated using the DFT calculation method. Two possible reaction mechanisms have been investigated: one that starts with oxidative addition of the indole (indole-first) or one that starts with oxidative cyclization of the 1,6-enyne (enyne-first). The computational results show that the enyne-first mechanism is more energetically favorable than the indole-first mechanism. The enyne-first mechanism contains several steps: pre-coordination, oxidative cyclization, C–H bond metathesis, and reductive elimination. Based on the calculations, the oxidative cyclization and the C–H bond metathesis are the stereoselectivity-determining processes, with the R-configured product being generated preferentially. The key to inducing the stereoselectivity is the less distortion of two interactive partners observed in the lower-energy transition state.


 Please wait while we load your content...
Please wait while we load your content...