Interactions between CO oxidation and selective catalytic reduction of NO with NH3 over Mn-based catalysts†
Abstract
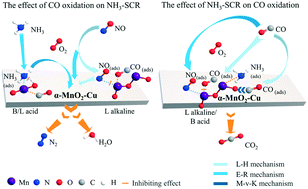
The simultaneous reaction of CO oxidation and selective catalytic reduction of NOx with NH3 (NH3-SCR) is of great challenge in heterogeneous catalysis, especially at low temperatures, and the interaction between these two reactions is not clear. In this work, a series of α-MnO2-M (M = Fe, Co, Ce, Cu) nanorod catalysts were synthesized by a hydrothermal method. The α-MnO2-Cu nanorod catalyst showed the highest catalytic performance with over 85% NOx and CO removal efficiency from 150 to 250 °C. The addition of Cu species increased the redox ability, total oxygen storage, surface acidic sites and total CO adsorption capacity, which should be responsible for the enhanced SCR and CO oxidation activity of the α-MnO2-Cu nanorod catalyst. The interaction of CO oxidation and SCR reaction has been investigated over the α-MnO2-Cu nanorod catalyst. CO reduced the NO adsorption, which further inhibited the L–H mechanism, reduced the SCR activity and narrowed the temperature window, especially the reduction of N2 selectivity. NH3 negatively affected CO oxidation by reducing the generation of the Mn–COO formate intermediate, while NO promoted CO oxidation through the decomposition of intermediates, which was formed by the combination of nitrate and nitrite with gaseous CO. When NH3 and NO existed together, CO oxidation was dominated by the promotional effect of NO.


 Please wait while we load your content...
Please wait while we load your content...