Iron molybdate catalysts synthesised via dicarboxylate decomposition for the partial oxidation of methanol to formaldehyde†
Abstract
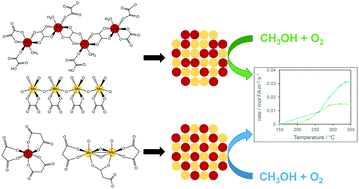
A series of iron molybdate catalysts were synthesised via a sol gel route using either oxalic acid or malonic acid. Catalysts synthesised using malonic acid were found to give improved formaldehyde yields over those prepared using oxalic acid or a standard coprecipitation method. This was attributed to the iron and molybdenum malonate precursors forming discrete ions that when precipitated gave a homogeneous distribution of iron and molybdenum in the final catalyst. Metal oxalate precursors and materials synthesised using coprecipitation gave less homogeneous structures containing iron rich centres that led to combustion of methanol to carbon oxides.


 Please wait while we load your content...
Please wait while we load your content...