Enantioselective Michael addition of aldehydes to maleimides catalysed by surface-adsorbed natural amino acids†‡
Abstract
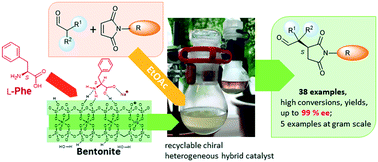
Asymmetric Michael addition of carbonyl compounds to N-substituted maleimides is an important method for obtaining optically pure succinimides, which are important chiral fine chemical intermediates. Environmentally friendly and sustainable procedures require the use of a heterogeneous, recyclable catalyst obtained from natural chirality sources and cheap auxiliaries. Here we report the application of in situ formed chiral inorganic–organic hybrid catalysts using amino acids such as L-phenylalanine and clay minerals or alumina, which were highly active and provided excellent enantioselectivities, up to 99%, in the addition of aldehydes to a large variety of N-substituted maleimides. Examinations indicated the occurrence of the asymmetric reaction on the surface of the recyclable solid hybrid materials. The catalytic materials were examined by thermogravimetry, XRD, FT-IR and Raman spectroscopy, SEM and adsorption experiments. Results of these methods showed that the amino acid is deposited as surface crystallites or intercalated in the layered cation exchangers, which both function as a supply of the chirality source, whereas the reactions are catalysed by the chiral compounds adsorbed on the surface. This catalytic system was used to conveniently prepare chiral succinimides at the gram scale, easily purified by crystallization. Accordingly, these chiral hybrid materials are convenient heterogeneous catalysts for obtaining valuable compounds in high optical purities using natural chirality sources, inorganic solids and ethyl acetate, a green organic solvent.


 Please wait while we load your content...
Please wait while we load your content...