Palladium-catalyzed synthesis of mixed anhydrides via carbonylative telomerization†
Abstract
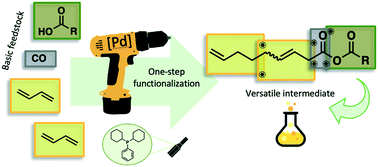
For the first time, mixed carboxylic anhydrides were accessed directly via homogeneous palladium catalysis from 1,3-butadiene and carboxylic acids. Under carbonylative telomerization conditions, the respective mixed 3,8-nonadienoic anhydrides are formed in a single reaction step with yields of up to 82%. These very reactive mixed anhydrides can then be used for consecutive reactions in a one-pot manner and selectively transfer the newly formed unsaturated C9 unit. Possible changes in the proposed mechanism were discussed and in a first example, the mixed anhydrides were utilized to form amides.


 Please wait while we load your content...
Please wait while we load your content...