Using molecular oxygen and Fe–N/C heterogeneous catalysts to achieve Mukaiyama epoxidations via in situ produced organic peroxy acids and acylperoxy radicals†
Abstract
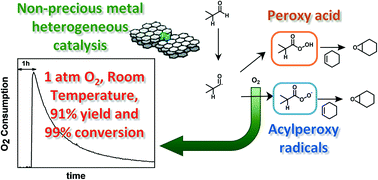
Under mild conditions of room temperature and pressure, and using either pure oxygen or air, aldehydes are converted using a heterogeneous Fe–N/C catalyst to produce the corresponding organic peroxy acid and acylperoxy radicals, which forms the epoxide from cyclohexene with high yield (91% for isobutyraldehyde in O2). Real-time monitoring of the rate of oxygen consumption and the electrochemical potential of the Fe–N/C catalyst has been used to study the formation of the peroxy acid and subsequent catalytic epoxidation of cyclohexene. Using isobutyraldehyde, it is shown that the aldehyde and the iron-based carbon catalyst (Fe–N/C) are involved in the rate determining step. Addition of a radical scavenger increases the induction time showing that radicals are initiated by the reaction between the aldehyde and the catalyst. Furthermore, UV-vis spectroscopy with 2,2′-azino-di-(3-ethylbenzthiazoline sulfonic acid) (ABTS) proved the in situ formation of peroxy acid. In the presence of cyclohexene, the peroxy acid leads to the corresponding epoxide with high yield. Monitoring the open circuit potential (OCP) and oxygen flow concurrently follows the production of the peroxy acid. The epoxidation reaction can take place only when the increase in open circuit potential is greater than 0.14 V, suggesting an in situ direct link between the relative oxidative strength of the peroxy acid and the likelihood of epoxidation.


 Please wait while we load your content...
Please wait while we load your content...