Electroreduction of NO3− on tubular porous Ti electrodes†
Abstract
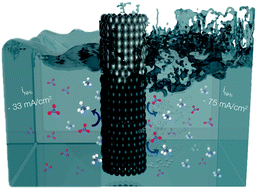
Inefficient fertilizer use in agriculture causes nitrate runoff, polluting rivers and streams. This pollution can be mitigated by partially converting nitrate into ammonia – rebalancing the composition to ammonium nitrate, and allowing recycling of fertilizer. Here, we present efficient electrochemical conversion of nitrate (50 mM) to ammonia in acidic electrolyte using tubular porous Ti electrodes. A high faradaic efficiency (FE) of 58% and partial current density to ammonia of −33 mA cm−2 at −1 V vs. RHE were achieved in the absence of inert gas purge. Additionally, we reveal that hydroxylamine is formed, as well as NO and N2O by spontaneous decomposition of nitrite, as has been determined by EC-MS analysis. The effective increase in local mass transport by introducing a flow of inert gas exiting the wall of the hollow fiber electrode results in an unprecedently high partial current density to ammonia of ∼−75 mA cm−2, while maintaining a faradaic efficiency to ammonia of up to 45%. This concept facilitates nitrate conversion at high FE even at low concentrations, and holds promise for development to practical scale if electrochemical potential and exiting gas flow rate are well controlled.


 Please wait while we load your content...
Please wait while we load your content...