Manganese-catalyzed transfer semihydrogenation of internal alkynes to E-alkenes with iPrOH as hydrogen source†
Abstract
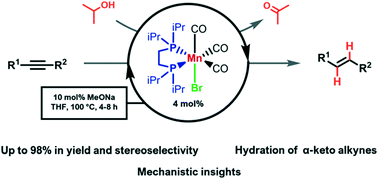
We report herein the homogeneous manganese catalyzed transfer semihydrogenation of internal alkynes to E-alkenes using iPrOH as the hydrogen source. Manganese-based catalytic precursors of the type fac-[Mn(X)(R2P(CH2)2PR2)(CO)3] (X = H, Br, and OTf; R = iPr (dippe), Cy (dcype), and Ph (dppe)) were tested and fac-[Mn(Br)(dippe)(CO)3] (Mn-2) exhibited the best catalytic activity, in the presence of MeONa (10 mol%), for the highly stereoselective transfer semihydrogenation of diphenylacetylene toward trans-stilbene. Different alkynes were assessed at the optimized conditions, obtaining from modest to good conversions and stereoselectivities. The best results were obtained with diaryl alkynes, while terminal alkynes and those with potential coordinative functional groups (e.g., alcohols and amines) remained unhydrogenated. Mn-catalyzed alkyne hydration was observed on using α-keto alkynes, resulting in 1,3-dicarbonylic compounds and derivates. Mechanistic studies were carried out, suggesting an inner-sphere mechanism with reversible alkene isomerization.


 Please wait while we load your content...
Please wait while we load your content...