Unraveling the mechanism of enantio-controlling switches of an alcohol dehydrogenase toward sterically small ketone†
Abstract
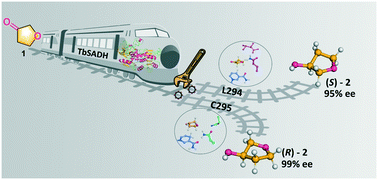
Efficient synthesis of chiral compounds under mild conditions is highly desirable in the chemical and pharmaceutical communities, but it often faces difficulties. Although various enzymes have been harnessed as biocatalysts in asymmetric reduction, identifying key residues that function as stereoselectivity-switches at which amino acid exchange events can be designed by rational design or directed evolution, constitutes a major challenge. Here, we address this question by using the Zn-dependent alcohol dehydrogenase from Thermoanaerobacter brockii (TbSADH) as a model enzyme in the asymmetric reduction of a difficult-to-reduce ketone (tetrahydrofuran-3-one). Following the theoretical analysis of epistatic mutational effects and investigating the results of deconvoluting multi-mutational variants, two crucial amino acid positions 294 and 295 were identified as the “switches” that specifically control the inversion of product chirality. In order to shed light on the concealed mechanistic intricacies, molecular dynamics (MD) simulations along with quantum mechanical (QM) calculations were performed on the variants, which unveiled the distinct H-bonds formed with the furan ring O-atom in pro-(S) and pro-(R) orientations of positions 294 and 295 leading to stereospecific formation of the product.


 Please wait while we load your content...
Please wait while we load your content...