Titania-supported molybdenum oxide combined with Au nanoparticles as a hydrogen-driven deoxydehydration catalyst of diol compounds†
Abstract
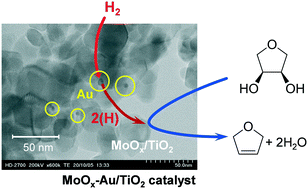
A heterogenous catalyst for the deoxydehydration (DODH) reaction was developed using less expensive Mo than Re as the active center. The combination of Mo with anatase-rich TiO2 and Au as the support and promoter for H2 activation, respectively, can selectively convert 1,4-anhydroerythritol to 2,5-dihydrofuran, which is a typical DODH model reaction, with H2 as a reducing agent. Loading of Au on TiO2 by the deposition–precipitation method gave the more active MoOx–Au/TiO2 catalyst (MoOx–dpAu/TiO2) than that obtained by the impregnation method (MoOx–impAu/TiO2), and the activity difference is derived from the smaller size of Au particles in MoOx–dpAu/TiO2 (3–5 nm) than that in MoOx–impAu/TiO2 (>25 nm). The MoOx–dpAu/TiO2 catalyst could be applied to the DODH reaction of linear alkyl vicinal diols and cis-1,2-cyclohexanediol. The characterization with XRD, STEM, H2-TPR, XAFS and XPS revealed that the MoIV oxide cluster species on the surface of anatase TiO2 particles are responsible for the DODH reaction.


 Please wait while we load your content...
Please wait while we load your content...