Advances in Diels–Alder/aromatization of biomass furan derivatives towards renewable aromatic hydrocarbons
Abstract
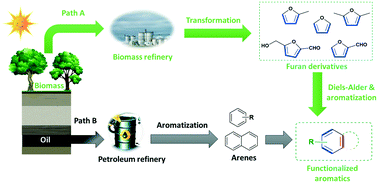
The effective upgrading of renewable resources into high value-added chemicals is of great significance to achieve sustainable economic development as well as the practical implementation of carbon neutral technologies. Diels–Alder (DA)/aromatization of biobased furan derivatives is being deemed as a major alternative to the process of producing petroleum-based chemicals from fossil carbon, given the high selectivity and efficient conversion of carbon atoms during the DA reaction. This mini-review focuses on the state-of-the-art progress regarding the DA strategy of bio-based furans. Of particular interest is to describe the research developments and future challenges in the production of renewable aromatic hydrocarbons by DA/aromatization of furan derivatives (furan, 2-methylfuran (MF), 2,5-dimethylfuran (DMF), furfural (FF), 5-hydroxymethylfurfural (HMF) and alkenes/alkynes, among many others. The effect of substituent properties on the reactivity of furan dienes in the cycloaddition process is discussed in detail, followed by analyzing the activation strategy of electron-poor furan derivatives (FF and HMF) as a diene in the DA reaction. Considering the experimental and calculation results, the DA/aromatization mechanisms of bioderived furan derivatives are also further depicted with paramount importance for acquiring an in-depth understanding while emphasizing the dominant existing challenges and future perspectives.


 Please wait while we load your content...
Please wait while we load your content...