Heterogenisation of a carbonylation catalyst on dispersible microporous polymer nanoparticles†
Abstract
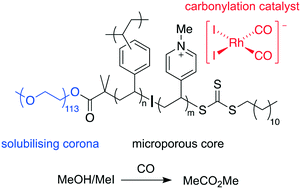
The methanol carbonylation catalyst, cis-[Rh(CO)2I2]−, has been heterogenised within a dispersible microporous polymer support bearing cationic functionality. The microporous polymer has a core–shell structure in which the porous and insoluble core (a cross-linked co-polymer of divinylbenzene and 4-vinylpyridine) is sterically stabilised by long hydrophilic poly(ethylene glycol) chains, allowing a stable nanoparticle dispersion to form. Incorporation of 4-vinylpyridine as a co-monomer allows post-synthetic modification to generate N-methylpyridinium sites for electrostatic attachment of the anionic rhodium(I) complex. The dispersibility of the polymer-supported catalyst material facilitates the use of in situ transmission IR spectroscopy to obtain kinetic data for the oxidative addition of iodomethane to immobilised cis-[Rh(CO)2I2]− (the rate-limiting step of the carbonylation cycle). The kinetic data followed a double exponential decay, with an initial fast phase having a rate constant k21 that is ∼5× greater than k22 for the slow phase (where k22 is similar to the homogeneous system using N-methylpyridinium counter-ions, CH2Cl2, 25 °C). The polymer-supported catalyst was found to be active for carbonylation of MeOH/MeI to give methyl acetate, with an initial rate ca. double that of the homogeneous analogue under the same conditions (10 bar CO, MeI/MeOH, 120 °C). Activity is lost over longer periods due to leaching of Rh from the support.


 Please wait while we load your content...
Please wait while we load your content...