Engineering the geometric and electronic structure of Ru via Ru–TiO2 interaction for enhanced selective hydrogenation†
Abstract
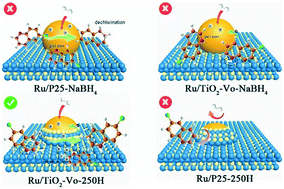
Modulation of the metal–support interaction plays a key role in many important chemical reactions. Here, by adjusting the reduction method of the catalyst and introducing oxygen vacancies in TiO2 to regulate the interaction between Ru and TiO2, four supported Ru nanocatalysts with different encapsulation degrees and electronic structures were obtained. Ru nanoparticles (NPs) partially encapsulated by TiO2 can achieve the selective hydrogenation of 6-chloroquinoline even at room temperature, with a TOF of 12 h−1. Catalytic characterization and DFT calculations indicated that partially encapsulated Ru NPs not only provided active sites for H2 dissociation, but also reduced the probability of Ru NPs being poisoned. Meanwhile, the oxygen vacancies on the surface of TiO2 can adsorb 6-chloroquinoline molecules and provide additional active sites for hydrogenation via hydrogen spillover. Moreover, the enhanced electron transfer from oxygen-deficient TiO2 to Ru made Ru electron-rich, which repelled C–Cl bonds and effectively prevented the production of dechlorination products.


 Please wait while we load your content...
Please wait while we load your content...