Formic acid generating in situ H2 and CO2 for nitrite reduction in the aqueous phase†
Abstract
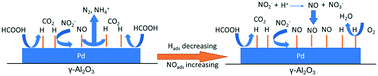
The aim of this work is to explore and to understand the effect of pH, concentrations and presence of oxygen traces on the reduction of nitrite in drinking water with Pd/γ-Al2O3, using formic acid as an in situ hydrogen supplier. Formic acid can reduce nitrite in the pH range between 4.5 and 8, producing negligible amounts of ammonium. By investigating the effect of pH, traces of oxygen and formic acid concentration on the conversion rates of both formic acid and nitrite, it is found that both the rate of conversion on nitrite with formic acid and the rate of formic acid decomposition are controlled by competitive adsorption of nitrite and formic acid on Pd, forming chemisorbed NO and chemisorbed H, respectively. The adsorbed species are studied with ATR-IR spectroscopy. Formic acid decomposition requires an ensemble of empty sites, favored by a low surface coverage of NO. The NO surface coverage, on the other hand, decreases with increasing hydrogen coverage, by converting NO to N2. The H-coverage in turn depends on the rate of formic acid decomposition. This causes an apparent order for the rate of formic acid decomposition of 1.4 in formic acid. In short, the surface coverage of NO should not be too high in order to have sufficient empty sites available for formic acid decomposition. When the pH of the solution is below 4.5, homogeneous disproportionation of nitric acid occurs forming nitrate and NO, resulting in catalyst poisoning with NO. The catalyst shows no activity at pH above 8, as formate ions are not reactive under such conditions.


 Please wait while we load your content...
Please wait while we load your content...