Enhanced oxygen reduction activity of size-selected platinum subnanocluster catalysts: Ptn (n = 3–9)†
Abstract
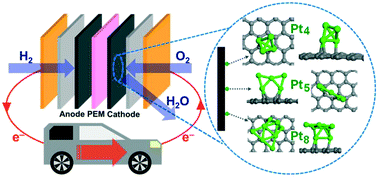
Nanoclusters (NCs) are promising candidates to improve catalytic activity despite the controversial size specificity, because atomicity is a crucial parameter that determines the activity of platinum (Pt) NCs. This paper demonstrates the enhanced catalytic activity based on the charge redistribution in Pt sub-NCs containing three to nine Pt atoms (Ptn; n = 3–9) on a glassy carbon substrate. The sub-NCs show 1.6–2.2 times higher activity than the standard Pt/C catalysts with a Pt crystallite diameter of 2 nm. The geometric structures are identified using rigorous structure analyses by X-ray absorption fine structure spectroscopy and density functional theory, and the activity origin within the supported Pt sub-NCs is theoretically discussed from viewpoints of energetics for reaction intermediates in the electrochemical processes. It should be possible to use sub-NCs in future fuel cell technologies as an active catalyst with a high atomic efficiency.


 Please wait while we load your content...
Please wait while we load your content...