Recent advances in subphthalocyanines and related subporphyrinoids†
Abstract
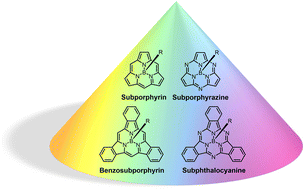
Half a century after the synthesis of the first subporphyrinoid, the study of tripyrrole and trisoindole porphyrin analogues constitutes a fervent and rapidly expanding research area. The outstanding structural, electronic and optical features of these cone-shaped aromatic macrocycles render them attractive candidates for a wide variety of applications, ranging from optoelectronics to biomedicine. To tune their properties and exploit their functionalities, the development of novel methodologies for the synthesis and post-functionalization of these contracted porphyrinoids, as well as a deep understanding of their supramolecular organization and their implementation into multicomponent systems of increasing complexity are of paramount importance. Herein, a review of the most recent advances in the fundamentals and applications of subporphyrinoids is presented, which comprehensively cover the last decade of discoveries. The final aim is to highlight the chemical versatility and intriguing physicochemical features of subporphyrinoids, while providing an updated overview of their most promising applications.
- This article is part of the themed collection: Trends and Challenges in Porphyrinoid Chemistry


 Please wait while we load your content...
Please wait while we load your content...