Electro-/photocatalytic alkene-derived radical cation chemistry: recent advances in synthetic applications
Abstract
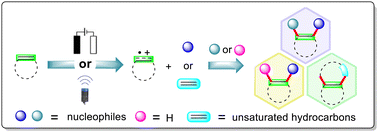
Alkene-derived radical cations are versatile reactive intermediates and have been widely applied in the construction of complex functionalized molecules and cyclic systems for chemical synthesis. Therefore, the synthetic application of these alkene-derived radical cations represents a powerful and green tool that can be used to achieve the functionalization of alkenes partially because the necessity of stoichiometric external chemical oxidants and/or hazardous reaction conditions is eliminated. This review summarizes the recent advances in the synthetic applications of the electro-/photochemical alkene-derived radical cations, emphasizing the key single-electron oxidation steps of the alkenes, the scope and limitations of the substrates, and the related reaction mechanisms. Using electrocatalysis and/or photocatalysis, single electron transfer (SET) oxidation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the alkenes occurs, generating the alkene-derived radical cations, which sequentially enables the functionalization of translocated radical cations to occur in two ways: the first involves direct reaction with a nucleophile/radical or two molecules of nucleophiles to realize hydrofunctionalization, difunctionalization and cyclization; and the second involves the transformation of the alkene-derived radical cations into carbon-centered radicals using a base followed by radical coupling or oxidative nucleophilic coupling.
C bonds in the alkenes occurs, generating the alkene-derived radical cations, which sequentially enables the functionalization of translocated radical cations to occur in two ways: the first involves direct reaction with a nucleophile/radical or two molecules of nucleophiles to realize hydrofunctionalization, difunctionalization and cyclization; and the second involves the transformation of the alkene-derived radical cations into carbon-centered radicals using a base followed by radical coupling or oxidative nucleophilic coupling.


 Please wait while we load your content...
Please wait while we load your content...