Insights into the binding of arginine to adenosine phosphate from mimetic complexes†
Abstract
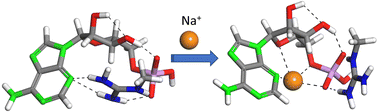
The amino acid arginine plays a key role in the interaction of proteins with adenosine phosphates, as its protonated guanidinium side group is capable of building multipodal H-bonding interactions with the oxygen atoms of the phosphate, phosphoester and ribose moieties and with the nitrogen atoms of adenine. Protein interactions often take place in competition with other ionic species, typically metal cations, which are prone to build concerted coordination arrangements with the same centers of negative charge as guanidinium. We report on a vibrational spectroscopy and computational investigation of a positively charged ternary complex formed by adenosine monophosphate (AMP) with methyl guanidinium and Na+. Following a bottom-up approach, an analogous complex with ribose phosphate is characterized as well, which serves to assess the individual role of the phosphate, sugar and adenine moieties in the binding process and to compare, within a single complex, the interactions associated with diffuse versus localized charge distributions of guanidinium and the alkali cation, respectively. The results indicate that Na+ is preferentially hosted in a semi-rigid pocket formed by the phosphoester–adenosine backbone of AMP and displaces guanidinium to a peripheral binding to the phosphate anionic end group. This suggests that the control of the salt concentration may constitute an effective route to modulate protein–AMP complexation.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...