P-block atom modified Sn(200) surface as a promising electrocatalyst for two-electron CO2 reduction: a first-principles study†
Abstract
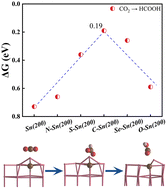
Low activity and poor product selectivity of CO2 reduction have seriously hampered its further practical application. Introducing p-block atoms to the catalyst is regarded as a promising strategy due to the versatility of p orbitals and diversity of p-block elements. Here, we systematically studied the influence of p-block atom X (X = C, N, O, S, and Se) on CO2 catalytic properties on a Sn(200) surface by first-principles calculation. Our work shows that all the p-block atoms are relative stable with Ef in the range of −5.11 to −3.59 eV. Further calculation demonstrates that the diversity of the p-block atoms results in unique CO2 electrocatalytic activity and product selectivity. Interestingly, the p-block C atom shows bi-functional activity to form two-electron products HCOOH and CO, with the corresponding energy barriers remarkably low at about 0.19 eV and 0.28 eV. In particular, the p-block S(Se) atom appears to have striking HCOOH selectivity, with the energy barrier to form HCOOH only a quarter of that to form the CO product. This unusual behavior is mainly attributed to the adsorption strength and frontier orbital interaction between the p-block atom and intermediates. These findings can effectively provide a valuable insight into the design of highly efficient CO2 electrocatalyst.


 Please wait while we load your content...
Please wait while we load your content...