Physical and numerical aspects of sodium ion solvation free energies via the cluster-continuum model†
Abstract
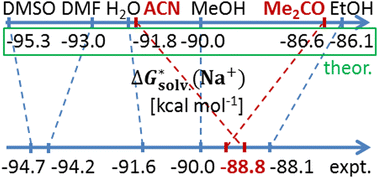
Sodium cation solvation Gibbs free energies (ΔGsolv(Na+)) have been obtained in water, dimethylformamide, dimethyl sulfoxide, ethanol, acetone, acetonitrile, and methanol through the “monomer cycle” cluster-continuum approach where a solvent reference state is described by infinitely separated molecules. The following steps are vital for obtaining reliable ΔGsolv(Na+) values: (a) a meticulous conformational search involving dispersion corrected density functional theory (DFT-D) and the continuum solvation model (CSM); (b) gas-phase DFT-D geometry optimization followed by single-point (SP) domain-based local pair natural orbital coupled clusters including single, double, and partly triple excitation (DLPNO-CCSD(T)) calculations in conjunction with the complete basis set extrapolation; (c) advanced statistical thermodynamic treatment of the low harmonic frequencies (<100 cm−1) to obtain the robust gas-phase Gibbs free energy correction; (d) gas-phase and dielectric continuum SP with non-electrostatic contributions included in the CSM; (e) an evaluation of the relative thermodynamic stability of the Na+(S)n clusters to identify the number of explicit solvent molecules n to be considered. Our refined computational protocol is promising with a Pearson correlation coefficient between the predicted and experimental data, ρ, of 0.82, and the mean signed and mean unsigned errors of 0.3 and 1.4 kcal mol−1, respectively.


 Please wait while we load your content...
Please wait while we load your content...