Carboxylate binding prefers two cations to one†
Abstract
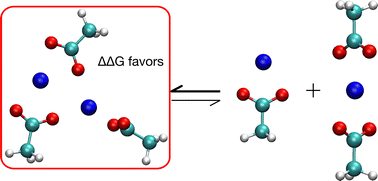
Almost all studies of specific ion binding by carboxylates (–COO−) have considered only a single cation, but clustering of ions and ligands is a common phenomenon. We apply density functional theory to investigate how variations in the number of acetate ligands in binding to two monovalent cations affects ion binding preferences. We study a series of monovalent (Li+, Na+, K+, Cs+) ions relevant to experimental work on many topics, including ion channels, battery storage, water purification and solar cells. We find that the preferred optimal structure has 3 acetates except for Cs+, which has 2 acetates. The optimal coordination of the cation by the carboxylate O atoms is 4 for both Na+ and K+, and 3 for Li+ and Cs+. There is a 4-fold coordination minimum just a few kcal mol−1 higher than the optimal 3-fold structure for Li+. For two cations, multiple minima occur in the vicinity of the lowest free energy state. We find that, for Li, Na and K, the preferred optimal structure with two cations is favored over a mixture of single cation complexes, providing a basis for understanding ionic cluster formation that is relevant for engineering proteins and other materials for rapid, selective ion transport.


 Please wait while we load your content...
Please wait while we load your content...