Structure-based thermodynamics of ion selectivity (Mg2+versus Ca2+ and K+versus Na+) in the active site of the eukaryotic lariat group II intron from algae Pylaiella littoralis†
Abstract
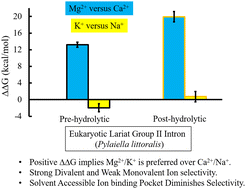
Group II introns are metalloenzymes that can catalyze self-splicing. Recently, the crystal structures of the eukaryotic group IIB lariat intron from the brown algae Pylaiella littoralis have been reported for two intermediate states (pre-hydrolytic (2s) and post-hydrolytic) along the self-splicing pathway. Three characteristic metal-ion binding sites (M1 and M2 sites for catalytic Mg2+ ions, and K1 site for K+) in the catalytic pocket of the lariat intron have been identified and proposed to be crucial for self-splicing. Using the X-ray structures as a template, we quantitatively estimated the energetics of divalent (Mg2+versus Ca2+) and monovalent (K+versus Na+) ion selectivity and established a direct link between the energetics and structures of this lariat intron (bound to cognate and near-cognate metal ions). Molecular dynamics (MD) free energy simulations showed that the lariat intron was strongly selective between divalent metal ions. The strength of divalent metal-ion selectivity was noticeably high in the post-hydrolytic state (ΔΔG ≈ 20 kcal mol−1) relative to its pre-hydrolytic (2s) state (ΔΔG ≈ 13 kcal mol−1). Quantum chemical calculations ensured that the sign of the estimated divalent metal-ion selectivity was correct. The M1-binding pocket was less solvent-exposed in the case of the post-hydrolytic state relative to the pre-hydrolytic (2s) state, which boosted the metal-ion selectivity of the former. Surprisingly, in contrast to the bacterial linear group II intron, the lariat intron was found to be non-selective between monovalent ions (K+versus Na+). The interaction network in the first coordination shell of Ca2+ in the M1-binding pocket was different relative to Mg2+. Mg2+ substitution by Ca2+ resulted in the substitution of a single M1–RNA interaction by the M1–water interaction. In the pre-hydrolytic (2s) state, Ca2+ substitution completely disrupted the M1⋯5′-exon interaction; thus, the nature of the divalent metal ion is critical for catalysis. The interaction network in the M2 site was independent of the nature of the divalent metal ions (Mg2+ or Ca2+). The monovalent ion was loosely bound in the wet binding pocket (K1 site) of the lariat intron; thus, the substitution of K+ by Na+ could not significantly alter the free energy of the complex. The metal ion selectivity was dependent on the solvent accessibility of the metal-ion-binding-pocket, dry pocket enhanced the selectivity.


 Please wait while we load your content...
Please wait while we load your content...