Altered coordination in a blue copper protein upon association with redox partner revealed by carbon–deuterium vibrational probes†
Abstract
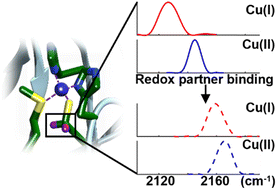
Proteins tune the reactivity of metal sites; less understood is the impact of association with a redox partner. We demonstrate the utility of carbon–deuterium labels for selective analysis of delicate metal sites. Introduced into plastocyanin, they reveal substantial strengthening of the key Cu-Cys89 bond upon association with cytochrome f.


 Please wait while we load your content...
Please wait while we load your content...