Monitoring MgCl2 hydrate formation from aqueous solutions using terahertz time-domain spectroscopy
Abstract
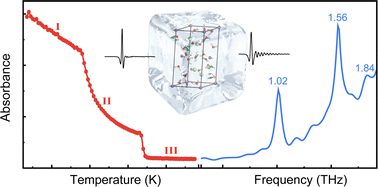
The interaction of MgCl2 with H2O is heavily involved in biological and chemical processes. In this work, freezing-induced hydrate formation from MgCl2 aqueous solution was monitored using terahertz time-domain spectroscopy. At low temperatures, two phase transitions from brine to hydrate formation could be clearly observed, and the formation of hydrate was accompanied by the emergence of new THz fingerprint peaks at 1.02, 1.56, and 1.84 THz, respectively. Integrating XRD and quantum chemical calculations, we attributed the absorption peaks to the vibrational modes of the formed MgCl2·12H2O. This demonstrates the potential of THz spectroscopy for application in the detection of biological processes in low-temperature environments, such as cell freezing.


 Please wait while we load your content...
Please wait while we load your content...