Hydration dynamics and IR spectroscopy of 4-fluorophenol†
Abstract
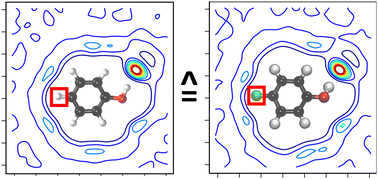
Halogenated groups are relevant in pharmaceutical applications and potentially useful spectroscopic probes for infrared spectroscopy. In this work, the structural dynamics and infrared spectroscopy of para-fluorophenol (F-PhOH) and phenol (PhOH) is investigated in the gas phase and in water using a combination of experiment and molecular dynamics (MD) simulations. The gas phase and solvent dynamics around F-PhOH and PhOH is characterized from atomistic simulations using empirical energy functions with point charges or multipoles for the electrostatics, Machine Learning (ML) based parametrizations and with full ab initio (QM) and mixed Quantum Mechanical/Molecular Mechanics (QM/MM) simulations with a particular focus on the CF- and OH-stretch region. The CF-stretch band is heavily mixed with other modes whereas the OH-stretch in solution displays a characteristic high-frequency peak around 3600 cm−1 most likely associated with the –OH group of PhOH and F-PhOH together with a characteristic progression below 3000 cm−1 due to coupling with water modes which is also reproduced by several of the simulations. Solvent and radial distribution functions indicate that the CF-site is largely hydrophobic except for simulations using point charges which renders them unsuited for correctly describing hydration and dynamics around fluorinated sites. The hydrophobic character of the CF-group is particularly relevant for applications in pharmaceutical chemistry with a focus on local hydration and interaction with the surrounding protein.


 Please wait while we load your content...
Please wait while we load your content...