Nature of hydrogen-bond-enhanced halogen bonding viewed through electron density changes†
Abstract
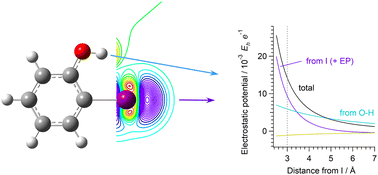
Elucidating the mechanism of how we can achieve fine tuning of intermolecular interaction strength will be helpful for designing functionally important molecules. In the present study, a theoretical analysis is conducted, by examining the electron density changes, for two halogen-bonding iodinated systems whose halogen-bond strengths have been considered to be enhanced by the presence of a hydrogen-bond donating group (termed hydrogen-bond-enhanced halogen bonding). It is shown that, contrary to the expectation obtained from the enhancement of electrostatic potential along the line extended from the C–I bond, the anisotropy of electron distribution on the iodine atom remains nearly the same. This means that the hydrogen bond and halogen bond contribute almost independently and additively to the enhancement of electrostatic potential, indicating the nature of this enhancement and, in a more general sense, the relationship between the strength and the extent of directionality of halogen bonding.


 Please wait while we load your content...
Please wait while we load your content...