Kinetic model for moisture-controlled CO2 sorption†
Abstract
The understanding of the sorption/desorption kinetics is essential for practical applications of moisture-controlled CO2 sorption. We introduce an analytic model of the kinetics of moisture-controlled CO2 sorption and its interpretation in two limiting cases. In one case, chemical reaction kinetics on pore surfaces dominates, in the other case, diffusive transport through the sorbent defines the kinetics. We show that reaction kinetics, which is dominant in the first case, can be expressed as a linear combination of 1st and 2nd order kinetics in agreement with the static isotherm equation derived and validated in a previous paper. The interior transport kinetics can be described by non-linear diffusion equations. By combining all carbon species into a single equation, we can eliminate – in certain limits – the source terms associated with chemical reactions. In this case, the governing equation is 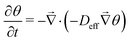 . For a sorbent in a form of a flat sheet or a membrane, one can maintain the same functional form of a diffusion equation by introducing a generalized effective diffusivity DM that combines contributions from both surface chemical reaction kinetics and interior diffusive transport kinetics. Experimental data of transient CO2 flux in a preconditioned commercial anion exchange membrane fit well to the 1st order model as long as very dry states are avoided, validating the theory. The observed DM for a preconditioned commercial anion exchange membrane ranges from 6.6 × 10−14 to 7.1 × 10−14 m2 s−1 at 35 °C. These small values compared to typical ionic diffusivities imply a very slow kinetics, which will be the largest issue that needs to be addressed for practical application. The collected transient CO2 flux data are used to predict the magnitude of a continuous CO2 pumping flux in an active membrane that transports CO2 against a CO2 concentration gradient. The pumped CO2 flux is supported by water flux due to a water concentration gradient.
. For a sorbent in a form of a flat sheet or a membrane, one can maintain the same functional form of a diffusion equation by introducing a generalized effective diffusivity DM that combines contributions from both surface chemical reaction kinetics and interior diffusive transport kinetics. Experimental data of transient CO2 flux in a preconditioned commercial anion exchange membrane fit well to the 1st order model as long as very dry states are avoided, validating the theory. The observed DM for a preconditioned commercial anion exchange membrane ranges from 6.6 × 10−14 to 7.1 × 10−14 m2 s−1 at 35 °C. These small values compared to typical ionic diffusivities imply a very slow kinetics, which will be the largest issue that needs to be addressed for practical application. The collected transient CO2 flux data are used to predict the magnitude of a continuous CO2 pumping flux in an active membrane that transports CO2 against a CO2 concentration gradient. The pumped CO2 flux is supported by water flux due to a water concentration gradient.



 Please wait while we load your content...
Please wait while we load your content...