Existence of noble gas-inserted phosphorus fluorides: FNgPF2 and FNgPF4 with Ng–P covalent bond (Ng = Ar, Kr, Xe and Rn)†
Abstract
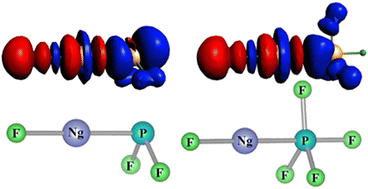
The scarce literature on noble gas (Ng)–phosphorous chemical bonding and our recent theoretical prediction of the FNgP molecule motivate us to explore a unique novel class of neutral noble gas-inserted phosphorus trifluoride and pentafluoride molecules, i.e., FNgPF2 and FNgPF4 (Ng = Ar, Kr, Xe, and Rn). The predicted molecules have been designed by inserting an Ng atom between the F and P atoms in the PF3 and PF5 molecules. The minima and saddle point geometries of all the FNgPFn (n = 2 and 4) molecules have been optimized using density functional theory (DFT) and second-order Møller–Plesset perturbation theory (MP2). The coupled cluster theory (CCSD(T)) method is also used to optimize the FNgPF2 molecules to test the performance of the above-mentioned methods. The predicted FNgPF2 and FNgPF4 molecules are found to be energetically stable with respect to all the probable 2-body and 3-body dissociation channels, except for the one leading to the global minimum products (Ng + PF3 and Ng + PF5). The existence of large barrier heights corresponding to the saddle point geometries is responsible for the kinetic stability of the metastable FNgPFn (n = 2 and 4) molecules, which prevents them from dissociating into their global minima products. The optimized structural parameters, energetics and harmonic vibrational frequency analysis suggest that the Ng–P bond is covalent in nature, while the F–Ng bond is mostly ionic in nature with some degree of covalency in the predicted molecules. In fact, the Ng–P bond length in the experimentally observed Ng–PF3 van der Waals complex is reduced significantly in the isomeric FNgPF2 molecule, almost leading to a conventional covalent Ng–P bond (cf. 4.152 vs. 2.413 Å for the Kr–P bond). Furthermore, the charge distribution and the AIM analysis also confirm the above-mentioned conclusion and indicate that the predicted FNgPF2 and FNgPF4 molecules can be represented as [F]δ−[NgPF2]δ+ and [F]δ−[NgPF4]δ+, respectively. All the computational results strongly reinforce the possible existence of these predicted FNgPFn (n = 2 and 4) molecules and clearly indicate that it may be possible to synthesize and characterize these molecules under suitable experimental technique(s).


 Please wait while we load your content...
Please wait while we load your content...