Theoretical study on Y-doped Na2ZrO3 as a high-capacity Na-rich cathode material based on anionic redox†
Abstract
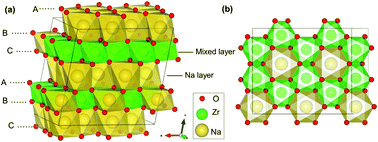
First-principles calculations based on density functional theory were utilized to study the performance of Na2ZrO3 (NZO) and yttrium-doped Na2ZrO3 (Y-NZO) as cathode materials for sodium ion batteries (SIBs), including the stability of the desodiated structures, desodiation energy, redox mechanism, and the diffusion of Na. When 62.5% sodium is removed from NZO, its structure and volume change little and the layered structure is retained, whereas the structure starts to distort and shift to the ZrO3 phase with the extraction of more than 62.5% sodium. As desodiation proceeds, oxygen anions act as the only redox center for charge compensation, yielding a high initial voltage of 4.03 eV vs. Na/Na+ by PBE + U-D3 functional and 4.82 eV vs. Na/Na+ by HSE06-D3 functional. When the desodiation content is less than 31.25%, O23− is formed with an O–O distance of 2.38 Å. At the desodiation content of 31.25%, peroxide dimer O22− starts to form; at the desodiation content of 56.25%, the O–O bond distance is further shortened to 1.3 Å, corresponding to the formation of superoxide O2−. However, for Y-NZO, the redox reaction firstly involves O2−/O1−, which does not occur in NZO. Peroxides and superoxides appear when the sodium removal concentration is 59.38% and 75%, respectively. This indicates that the O–O dimers appear in Y-NZO at much deeper sodium removal. The calculations of diffusion paths and barriers of Na ions in NZO by PBE + U-D3 predict that the barrier of Na escaping from the mixed layer to the Na layer in NZO is 0.48 eV (the reverse barrier is 0.76 eV), smaller than those of other O3-type layered transition metal compounds, such as Na2IrO3 and Na2RuO3. After yttrium doping, the diffusion of Na ions becomes easier, indicating that the Y-doping improves the diffusion ability. This investigation interprets the mechanism of oxygen oxidation of NZO as a cathode for SIBs, and provides theoretical support for a better design of Na-rich layered oxide Na2MO3 (M represents the transition metal element) in the future research.


 Please wait while we load your content...
Please wait while we load your content...