Structural and thermodynamic insights into the Cren7 mediated DNA organization in Crenarchaeota†
Abstract
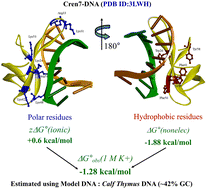
Archaea have histone homologues and chromatin proteins to organize their DNA into a compact form. This allows them to survive in extreme climates. Cren7 is one such chromatin protein conserved in Crenarchaeota. When Cren7 binds to model natural DNA, calf thymus DNA (CTD, 58% AT content) and polynucleotides under adverse solution conditions (high temperature, ionic strength), CD bands at 275–290 nm shift to higher wavelengths indicating structural changes in DNA. It formed a strong complex with CTD and poly(dA-dT)·poly(dA-dT), via a combination of electrostatic and non-electrostatic interactions. A low binding enthalpy indicated that the process was driven by entropy. The interaction was independent of the nature of the anions present in the solution. On studying the variation in protein affinity with salt concentration, it was estimated that the electrostatic interaction at the interface involves 3 pairs of ions at the protein–DNA interface. The affinity and binding site size decreased on changing the pH of the solution (between pH 6 and 8), but temperature did not result in such effects. Cren7 bound to 10 bp of DNA, increasing its flexibility and thermal stability by more than 30 °C. Increasing the amount of Cren7 produces cooperative structural transitions in DNAs without any similar transition in the protein. These crucial binding parameters, energetics, and structural changes decipher the mystery of Cren7 mediated DNA organization in Crenarchaeota.


 Please wait while we load your content...
Please wait while we load your content...