A powder XRD, solid state NMR and calorimetric study of the phase evolution in mechanochemically synthesized dual cation (Csx(CH3NH3)1−x)PbX3 lead halide perovskite systems†‡
Abstract
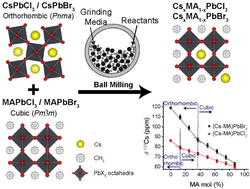
Methylammonium (MA+) lead halide perovskites (MAPbX3) have been widely investigated for photovoltaic applications, with the addition of Cs improving structural and thermal stability. This study reports the complete A site miscibility of Cs+ and MA+ cations in the lead chloride and lead bromide perovskites with nominal stoichiometric formulae (CsxMA1−x)Pb(Cl/Br)3 (x = 0, 0.13, 0.25, 0.37, 0.50, 0.63, 0.75, 0.87, 1). These suites of materials were synthesized mechanochemically as a simple, cost-effective synthesis technique to produce highly ordered, single phase particles. In contrast to previous studies using conventional synthetic routes that have reported significant solubility gaps, this solvent-free approach induces complete miscibility within the dual cation Cs+/MA+ system, with the resultant structures exhibiting high short-range and long-range atomic ordering across the entire compositional range that are devoid of solvent inclusions and disorder. The subtle structural evolution from cubic to orthorhombic symmetry reflecting PbX6 octahedral tilting was studied using complementary high resolution TEM, powder XRD, multinuclear 133Cs/207Pb/1H MAS NMR, DSC, XPS and UV/vis approaches. The phase purity and exceptional structural order were reflected in the very high resolution HRTEM images presented from particles with crystallite sizes in the ∼80–170 nm range, and the stability and long lifetimes of the Br series (10–20 min) and the Cl series (∼30 s–1 min) under the 200 kV/146 μA e− beam. Rietveld refinements associated with the room temperature PXRD study demonstrated that each system converged towards single phase compositions that were very close to the intended target stoichiometries, thus indicating the complete miscibility within these dual cation Cs+/MA+ solid solution systems. The multinuclear MAS NMR data showed a distinct sensitivity to the changing solid solution compositions across the MAPbX3–CsPbX3 partition. In particular, the 133Cs shifts demonstrated a sensitivity to the cubic–orthorhombic phase transition while the 133Cs T1s exhibited a pronounced sensitivity to the variable Cs+ cation mobility across the compositional range. Variable temperature PXRD studies facilitated the production of phase diagrams mapping the Cs+/MA+ compositional space for the (CsxMA1−x)PbCl3 and (CsxMA1−x)PbBr3 solid solution series, while Tauc plots of the UV/vis data exhibited reducing bandgaps with increasing MA+ incorporation through ranges of cubic phases where octahedral tilting was absent.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...