Study of the electrochemical betanidin oxidation path using computational methods†
Abstract
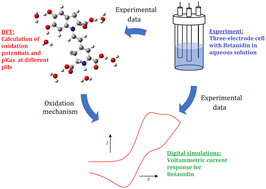
Betalains can be used in the food, drug, and cosmetic industries and have shown their bioactive potential. For these reasons, unraveling their oxidation mechanism is of high importance and demands a systematic and multidisciplinary study. Moreover, the properties mentioned above are drastically influenced by pH and other physicochemical conditions. Betanidin (1) is a relevant molecule of this family and is crucial to elucidating the oxidation mechanism in which its pigment is involved. In the present study, the pKas and oxidation potential 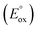 values for all protic groups of 1 were analyzed using B3LYP/6-31+G(d,p)/SMD as the computational methodology. Moreover, six explicit water molecules were added to improve the solvation-free energy values. The oxidation mechanism at each pH was constructed and analyzed in depth. On the other hand, cyclic voltammetry simulations allowed obtaining electrochemical data from experiments and support the proposed mechanism. In the present work, the main oxidation path of 1 is described and consists of a concerted electron–proton transfer followed by a sequential electron and proton transfer to obtain the o-quinone product or a quinone methide molecule.
values for all protic groups of 1 were analyzed using B3LYP/6-31+G(d,p)/SMD as the computational methodology. Moreover, six explicit water molecules were added to improve the solvation-free energy values. The oxidation mechanism at each pH was constructed and analyzed in depth. On the other hand, cyclic voltammetry simulations allowed obtaining electrochemical data from experiments and support the proposed mechanism. In the present work, the main oxidation path of 1 is described and consists of a concerted electron–proton transfer followed by a sequential electron and proton transfer to obtain the o-quinone product or a quinone methide molecule.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...