Interfacial charge transfer complex formation between silver nanoparticles and aromatic amino acids†
Abstract
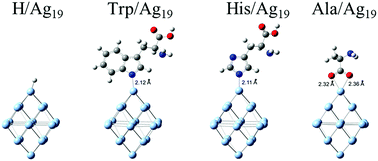
The optical properties of surface-modified silver nanoparticles (Ag NPs) with aromatic amino acids tryptophan (Trp) and histidine (His) were examined using the cluster model for density functional theory (DFT) and time-dependent density functional theory (TD-DFT) calculations. Also, the redistribution of electronic charges upon chemisorption of ligand molecules onto silver's surfaces is determined. The obtained theoretical data, on one side, undoubtedly indicate the the formation of an interfacial charge transfer (ICT) complex between silver and this type of ligand, and, on the other side, partial oxidation of surface silver atoms accompanied by an increase of electron density in ligand molecules. The ICT complex formation, based on noble metal nanoparticles, has never been reported previously to the best of our knowledge. The experimental spectroscopic measurements support the theoretical data. A new absorption band in the visible spectral range appears upon surface modification of Ag NPs, and, when exposed to air, oxidation of surface-modified Ag NPs is significantly faster than the oxidation of the unmodified ones.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...