Evidence suggesting kinetic unfreezing of water mobility in two distinct processes in pressure-amorphized clathrate hydrates†
Abstract
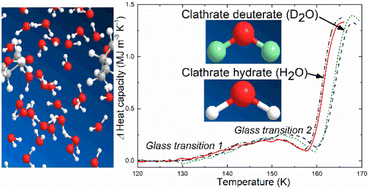
Type II clathrate hydrates (CHs) with tetrahydrofuran (THF), cyclobutanone (CB) or 1,3-dioxolane (DXL) guest molecules collapse to an amorphous state near 1 GPa on pressurization below 140 K. On subsequent heating in the 0.2–0.7 GPa range, thermal conductivity and heat capacity results of the homogeneous amorphous solid show two glass transitions, first a thermally weak glass transition, GT1, near 130 K; thereafter a thermally strong glass transition, GT2, which implies a transformation to an ultraviscous liquid on heating. Here we compare the GTs of normal and deuterated samples and samples with different guest molecules. The results show that GT1 and GT2 are unaffected by deuteration of the THF guest and exchange of THF with CB or DXL, whereas the glass transition temperatures (Tgs) shift to higher temperatures on deuteration of water; Tg of GT2 increases by 2.5 K. These results imply that both GTs are associated with the water network. This is corroborated by the fact that GT2 is detected only in the state which is the amorphized CH's counterpart of expanded high density amorphous ice. The results suggest a rare transition sequence of an orientational glass transition followed by a glass to liquid transition, i.e., kinetic unfreezing of H2O reorientational and translational mobility in two distinct processes.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...