Physico-geometrical kinetics of the thermal dehydration of sodium carbonate monohydrate as a compacted composite of inorganic hydrate comprising crystalline particles and matrix†
Abstract
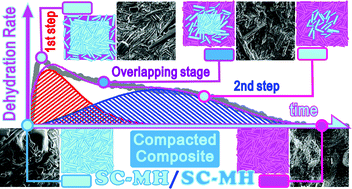
The kinetics of the thermal dehydration of compacted composite grains of Na2CO3·H2O (SC-MH) comprising columnar SC-MH crystalline particles and an SC-MH matrix were investigated as a model system for composites of the same compound with a porphyritic texture. The presence of an induction period was confirmed as a novel finding for the thermal dehydration of SC-MH. The subsequent mass loss process was characterized as a partially overlapping two-step process attributed to the consecutive reactions of SC-MH matrix and columnar SC-MH crystalline particles. The overlapping nature of two reaction steps was revealed by determining the contributions and kinetic parameters of the individual reaction steps via a kinetic deconvolution analysis. Furthermore, the initial mass loss process caused by the thermal dehydration of the SC-MH matrix was characterized as a physico-geometrical consecutive process comprising a surface reaction and a subsequent three-dimensional (3D)-phase boundary-controlled reaction. The subsequent thermal dehydration of the columnar SC-MH crystalline particles compacted in the grains was characterized as being geometrically constrained by 3D-interface shrinkage, forming two reaction interfaces during the overlapping stage of the two reaction steps. It was expected from the kinetic results that the linear advancement rate of the second reaction interface was influenced by the water vapor produced at the reaction interface of the first reaction step. This caused the linear advancement rate of the second reaction interface to accelerate as the reaction proceeded due to contraction of the first reaction interface and completion of the first reaction step.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...