A theoretical study of the role of the non-innocent phenolate ligand of a nickel complex in water oxidation†
Abstract
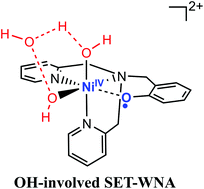
Water oxidation is the bottleneck of artificial photosynthesis. A novel nickel phenolate complex with a redox-active ligand has been designed to manage multiple electron transfers during water oxidation (D. Wang and C. O. Bruner, Inorg. Chem., 2017, 56, 13638). However, the mechanism of the reaction is not well understood and verified from a theoretical aspect. Density functional theory calculations were conducted to investigate the mechanism of water oxidation catalyzed by the nickel(II)–phenolate complex. Because only two cyclic voltammogram (CV) peaks were observed and the phenolate ligand is redox-active, the active species was proposed to be NiIII–OH by the experiment. Based on the calculated results, the first CV peak is phenolate ligand-centered and the second peak is a single two-proton-coupled-two-electron process. In addition, the activation barrier of O–O bond formation of NiIII–OH is higher than that of NiIV–2OH by 15.3 kcal mol−1. Thus, the redox-active phenolate ligand does not lower the oxidation state of Ni in the active species to NiIII. The oxidation state of the active species is still NiIV, the same as other Ni complexes for WOCs. As the phenolate ligand and the hydroxyl ligand can act as an internal base, three pathways are compared for O–O bond formation: normal WNA, phenolate-involving single electron transfer (SET)-WNA, and OH-involving SET-WNA. The OH-involving SET-WNA pathway is the most favorable because the hydroxyl ligand is more nucleophilic than the oxygen radical of the phenolate ligand. Based on the experimental observation and theoretical results, the phenolate ligand is not stable and easily oxidized because of the hydrogen at the benzyl position. Thus, WOC candidates should not have the presence of hydrogen at the benzyl position near the active center.


 Please wait while we load your content...
Please wait while we load your content...