A temperature programmed desorption study of interactions between water and hydrophobes at cryogenic temperatures
Abstract
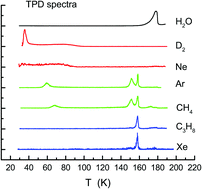
It is considered that hydrophobic solutes dissolve in water via the formation of icelike cages in the first hydration shell. However, this conventional picture is currently under debate. We have investigated how hydrophobic species, such as D2, Ne, Ar, Xe, CH4, and C3H8, interact with water in composite films of amorphous solid water (ASW) based on temperature programmed desorption (TPD). The D2 and Ne species tend to be incorporated in ASW without being caged, whereas two distinct peaks assignable to the caged species are identifiable for the other solutes examined here. The low-temperature peak is observed preferentially for Ar and CH4 prior to crystallization. The hydrophobes are thought to be encapsulated in porous ASW films via reorganization of the hydrogen bond network up to 100 K; most of them are released in a liquidlike phase that occurs immediately before crystallization at ca. 160 K. The nature of hydrophobic hydration at cryogenic temperature appears to differ from that in normal water at room temperature because the former resembles crystalline ices in the local hydrogen-bond structure rather than the latter. No ordered structures assignable to clathrate hydrates were identified before and after crystallization.


 Please wait while we load your content...
Please wait while we load your content...