Bimolecular reactions of CH2CN2+ with Ar, N2 and CO: reactivity and dynamics†
Abstract
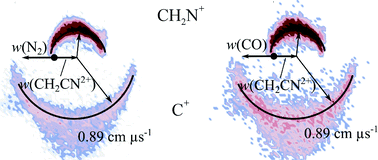
The reactivity, energetics and dynamics of bimolecular reactions between CH2CN2+ and three neutral species (Ar, N2 and CO) have been studied using a position sensitive coincidence methodology at centre-of-mass collision energies of 4.3–5.0 eV. This is the first study of bimolecular reactions involving CH2CN2+, a species relevant to the ionospheres of planets and satellites, including Titan. All of the collision systems investigated display two collision-induced dissociation (CID) channels, resulting in the formation of C+ + CH2N+ and H+ + HC2N+. Evidence for channels involving further dissociation of the CID product HC2N+, forming H + CCN+, were detected in the N2 and CO systems. Proton-transfer from the dication to the neutral species occurs in all three of the systems via a direct mechanism. Additionally, there are product channels resulting from single electron transfer following collisions of CH2CN2+ with both N2 and CO, but interestingly no electron transfer following collisions with Ar. Electronic structure calculations of the lowest energy electronic states of CH2CN2+ reveal six local geometric minima: both doublet and quartet spin states for cyclic, linear (CH2CN), and linear isocyanide (CH2NC) molecular geometries. The lowest energy electronic state was determined to be the doublet state of the cyclic dication. The ready generation of C+ ions by collision-induced dissociation suggests that the cyclic or linear isocyanide dication geometries are present in the [CH2CN]2+ beam.
- This article is part of the themed collection: Ions, electrons, coincidences and dynamics: Festschrift for John H.D. Eland


 Please wait while we load your content...
Please wait while we load your content...