An extensive theoretical study on the thermochemistry of aromatic compounds: from electronic structure to group additivity values†
Abstract
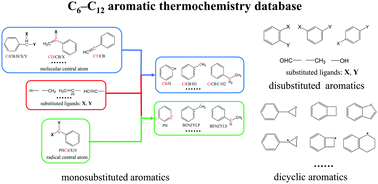
An extensive and reliable database of thermodynamic properties of C6–C12 aromatic molecules is constructed by using quantum chemistry calculations. There are 101 molecules in total, which cover a variety of structures including mono-substituted, di-substituted, and bi-cyclic aromatics which can be important intermediates in the combustion of alkylbenzenes. Based on the database, a consistent set of Benson group additive values (GAV) and non-nearest neighbor interactions (NNI) is developed to extend the applicability of Benson's group additivity method for aromatic molecules. Meanwhile, GAVs of existing groups are also updated to improve their accuracy. Geometry optimizations, and vibrational frequency calculations are conducted at the M06-2X/6-311++G(d,p) level of theory. Internal rotor potentials for lower-frequency modes are obtained at the M06-2X/6-31G level of theory. G3 and G4 compound methods are used to derive the 0 K enthalpies of formation via the atomization approach. The entropy and temperature-dependent heat capacity values of all species are calculated via the Master Equation System Solver (MESS) code. This work also provides an extensive literature comparison to validate the calculated results, and good agreement is observed with literature data. The correction terms beyond a group range are explored. The NNIs of di-substituted aromatics with substituents including OH, CHO, and CH3 groups are reported. Entropy reduction is observed in the molecules with two substituents in the ortho position, which mainly derives from the hindered internal rotations. In addition, ring strain corrections (RSC) of dicyclic aromatics are evaluated. The strain energies of molecules with a four-membered side ring are prominently large, as the bond length and bond angle distortions are severely restricted. Ring strain also plays a key role in the C–H bond strength associated with the benzylic carbons in dicyclic aromatics. The loss of a hydrogen atom can destroy the high ring-strain geometry leading to a large C–H bond energy.


 Please wait while we load your content...
Please wait while we load your content...