Li+ transference number and dynamic ion correlations in glyme-Li salt solvate ionic liquids diluted with molecular solvents†
Abstract
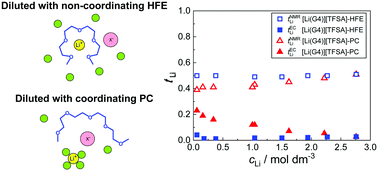
Highly concentrated electrolytes (HCEs) have attracted significant interest as promising liquid electrolytes for next-generation Li secondary batteries, owing to various beneficial properties both in the bulk and at the electrode/electrolyte interface. One particular class of HCEs consists of binary mixtures of lithium bis(trifluoromethanesulfonyl)amide (LiTFSA) and oligoethers that behave like ionic liquids. [Li(G4)][TFSA], which comprises an equimolar mixture of LiTFSA and tetraglyme (G4), is an example. In our previous works, the addition of low-polarity molecular solvents to [Li(G4)][TFSA] was found to effectively enhance the conductivity while retaining the unique Li-ion solvation structure. However, it remains unclear how the diluents affect another key electrolyte parameter—the Li+ transference number—despite its critical importance for achieving the fast charging/discharging of Li secondary batteries. Thus, in this study, the effects of diluents on the extremely low Li+ transference number under anion-blocking conditions in [Li(G4)][TFSA] were elucidated, with a special focus on the polarity of the additional solvents. The concentration dependence of the dynamic ion correlations was further studied in the framework of the concentrated electrolyte theory. The results revealed that a non-coordinating diluent is not involved in the modification of the ion transport mechanism, and therefore the low Li+ transference number is inherited by the diluted electrolytes. In contrast, a coordinating diluent effectively reduces the anti-correlated ion motions of [Li(G4)][TFSA], thereby improving the Li+ transference number. This is the first time that the significant effects of the coordination properties of the diluting solvents on the dynamic ion correlations and Li+ transference numbers have been reported for diluted solvate ionic liquids.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...