Quantum effects in photosensitization: the case of singlet oxygen generation by thiothymines†
Abstract
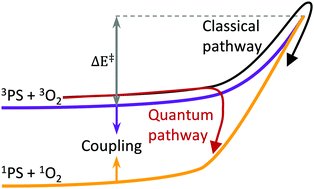
Photosensitization is the indirect electronic excitation of a molecule with the aid of a photosensitizer and is a bimolecular nonradiative energy transfer. In this study, we have attempted to elucidate its mechanism, and we do this by calculating rate constants of photosensitization of oxygen by thiothymines (2-thiothymine, 4-thiothymine and 2,4 dithiothymine). The rate constants have been calculated using two approaches: (a) a classical limit of Fermi's Golden Rule (FGR), and (b) a time-dependent variant of FGR, where the treatment is purely quantum mechanical. The former approach has previously been developed for bimolecular systems and has been applied to the photosensitization reactions studied here. The latter approach, however, has so far only been used for unimolecular reactions, and in this work, we describe how it can be adapted for bimolecular reactions. Experimentally, all three thiothymines are known to have significant singlet oxygen yields, which are indicative of similar rates. Rate constants calculated using the time-dependent variant of FGR are similar across all three thiothymines. While the classical approximation gives reasonable rate constants for 2-thiothymine, it severely underestimates them for 4-thiothymine and 2,4 dithiothymine, by several orders of magnitude. This work indicates the importance of quantum effects in driving photosensitization.


 Please wait while we load your content...
Please wait while we load your content...