Recipes for superior ionic conductivities in thin-film ceria-based electrolytes
Abstract
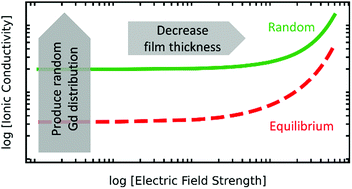
We employed Molecular Dynamics (MD) and Metropolis Monte Carlo (MMC) simulations to determine the oxide-ion mobility uO in Ce1−yGdyO2−y/2 (y = 0.02, 0.1, 0.2) for the range of temperatures 1400 ≤ T/K ≤ 2000 and field strengths 0.6 ≤ E/MV cm−1 ≤ 15.0. Direct, unambiguous determination of uO(E) from MD simulations is shown to require examination of the ions' mean displacement as a function of time. MD simulations were performed for random distributions of Gd cations and equilibrium distributions obtained by MMC calculations. All uO(E,T,y) data obtained can be described by an (empirically augmented) analytical model with four zero-field parameters, a result that allows data to be extrapolated down to the temperatures of electrolyte operation. Specifically, the oxide-ion conductivity is predicted, for example at T = 700 K, (i) to be up to 101 higher for a random distribution of Gd than for an equilibrium distribution; and (ii) to be a factor of 100.8 higher for a 6 nm thin film than for a μm-thick sample under a potential difference of 1 V. By virtue of non-equilibrium deposition and nm-thick samples, thin films thus provide two new recipes for attaining even higher oxide-ion conductivities in ceria-based electrolytes.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...