Sequence dependent folding motifs of the secondary structures of Gly-Pro and Pro-Gly containing oligopeptides†
Abstract
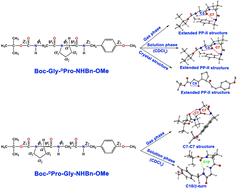
Folding motifs of the secondary structures of peptides and proteins are primarily based on the hydrogen bonding interactions in the backbone as well as the sequence of the amino acid residues present. For instance, the β-turn structure directed by the Pro-Gly sequence is the key to the β-hairpin structure of peptides/proteins as well as a selective site for the enzymatic hydroxylation of pro-collagen. Herein, we have investigated the sequence dependent folding motifs of end-protected Gly-Pro and Pro-Gly dipeptides using a combination of gas phase laser spectroscopy, quantum chemistry calculations, solution phase IR and NMR spectroscopy and single crystal X-Ray diffraction (XRD). All three observed conformers of the Gly-Pro peptide in the gas phase have been found to have extended β-strand or polyproline-II (PP-II) structures with C5–C7 hydrogen bonding interactions, which correlates well with the structure obtained from solution phase spectroscopy and XRD. On the other hand, we have found that the Pro-Gly peptide has a C10/β-turn structure in the solution phase in contrast to the C7–C7 (i.e. 27-ribbon) structure observed in the gas phase. Although the lowest energy structure in the gas phase is not C10, we find that C7–C7 is an abundantly found structural motif of Pro-Gly containing peptides in the Cambridge Structural Database, indicating that the gas phase conformers are not sampling any unusual forms. We surmise that the role of the solvent could be crucial in dictating the preferential stabilization of the C10 structure in the solution phase. The present investigation provides a comprehensive picture of the folding motifs of the Gly-Pro and Pro-Gly peptides observed in the gas phase and condensed phase weaving a fine interplay of the intrinsic conformational properties, solvation, and crystal packing of the peptides.


 Please wait while we load your content...
Please wait while we load your content...