Ab initio trajectory surface-hopping dynamics studies of excited-state proton-coupled electron transfer reactions in trianisoleheptazine–phenol complexes†
Abstract
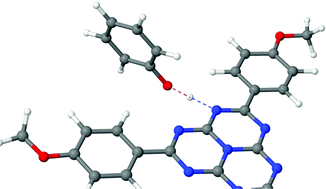
The excited-state proton-coupled electron-transfer (PCET) reaction in hydrogen-bonded complexes of trianisoleheptazine (TAHz), a chromophore related to polymeric carbon nitrides widely used in hydrogen-evolution photocatalysis, with several phenol derivatives were recently studied by Schlenker and coworkers with time-resolved photoluminescence quenching and pump–probe experiments. A pronounced dependence of the PCET reactivity on the electron-donating/electron-withdrawing character of the substituents on phenol was found, with indications of a barrierless or nearly barrierless PCET reaction for the most strongly electron-donating substituent, methoxy. In the present work, the excited-state PCET dynamics was explored with first-principles nonadiabatic dynamics simulations using the TDDFT/ωB97X-D electronic-structure model for two selected complexes, TAHz–phenol and TAHz–methoxyphenol. The qualitative reliability of the TDDFT/ωB97X-D electronic-structure model was assessed by extensive benchmarking of excitation energies and potential-energy profiles against a wave-function-based ab initio method, the algebraic-diagrammatic construction of second order (ADC(2)). The nonadiabatic dynamics simulations provide temporally and structurally resolved insights into paradigmatic PCET reactions in TAHz–phenol complexes. The radiationless relaxation of the photoexcited bright 1ππ* state to the long-lived dark S1 state of TAHz occurs in less than 100 fs. The ensuing PCET reaction on the adiabatic S1 surface is faster in TAHz–methoxyphenol complexes than in TAHz–phenol complexes due to a lower H-atom-transfer barrier, as observed in the experiments. The relaxation of the complexes to the electronic ground state is found to occur exclusively via PCET within the 250 fs time window covered by the present simulations, confirming the essential role of the hydrogen bond for the fluorescence quenching process. The absolute values of the computed PCET time constants are significantly shorter than those extracted from time-resolved photoluminescence measurements for mixtures of TAHz with phenolic substrates in toluene. The possible origins of this discrepancy are discussed.


 Please wait while we load your content...
Please wait while we load your content...