Protonation and orientation: a computational approach to cocaine diffusion through a model membrane†
Abstract
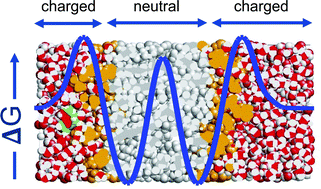
We study the diffusion of cocaine through a DMPC lipid bilayer as an example of a protonable, amphiphilic molecule passing a biological membrane. Using classical molecular dynamics simulations, the free energy surfaces are computed applying the umbrella sampling technique for the protonated and the neutral molecule. For the combined surface, we numerically solve the diffusion equation at constant flow and for time-dependent concentrations. We find a potential of mean force dominated by a barrier of 3.5 kcal mol−1 within the membrane, and a pH-dependent entry and exit barrier of 2.0 kcal mol−1 and 4.1 kcal mol−1, respectively. This behaviour can be rationalized chemically by the amphiphilic nature of the molecule and the change of its protonation state while passing the membrane. Diffusion through the barriers is 3.5 times slower than along the membrane, and the typical time scale of passage amounts to 0.1 ms. We discuss biochemical and medical implications of our findings, and comment on the mechanism of the drug passing the blood–brain barrier.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...