Estimation of the nucleation barrier in a multicomponent system with intermolecular potential
Abstract
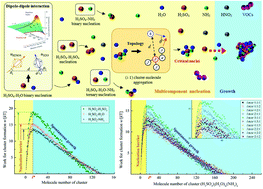
The formation of a “critical nucleus” prior to phase change is a crucial step for new particle formation (NPF) in the atmosphere. However, the nucleation occurring below ∼1 nm is hard to observe directly. As an effective alternative, theoretical nucleation models have been widely studied. An energy barrier is involved in the nucleation and is the fundamental factor for the nucleation model. Typical atmospheric nucleation agents such as H2SO4, H2O and NH3 are dipole molecules, whose intermolecular interactions are non-ignorable. Herein, a dipole–dipole potential model is adopted to determine the interaction between molecules instead of the traditional hard sphere model, and graph theory is used to describe the structure of the cluster and the cluster–molecule interaction. The nucleation barriers (ΔEb) of H2SO4–H2SO4, H2SO4–H2O, H2SO4–NH3 and H2SO4–H2O–NH3 are derived and compared to each other. In the presence of H2O and NH3, the ΔEb value is decreased by 17–28% compared to that in the pure H2SO4 nucleation system. NH3 is identified to be a key factor for ternary nucleation based on an orthogonal test. Atmospheric concentrations of H2SO4, H2O and NH3 are considered to investigate the influence of [H2O + NH3]/[H2SO4] on ΔEb and the related effective collision coefficient (α). The α value in the ternary nucleation system reaches the range of (2.5–25) × 10−5, which is 3–4 orders of magnitude higher than that in the pure H2SO4 system. Due to a significant enhancement of α, NH3 and H2O should be focused on in future aerosol particle estimation and control.


 Please wait while we load your content...
Please wait while we load your content...