Novel D–A chromophores with condensed 1,2,4-triazine system simultaneously display thermally activated delayed fluorescence and crystallization-induced phosphorescence†
Abstract
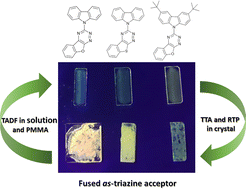
Control of photophysical properties is crucial for the continued development of electroluminescent devices and luminescent materials. Preparation and study of original molecules uncovers design rules towards efficient materials and devices. Here we have prepared 7 new compounds based on the popular donor–acceptor design used in thermally activated delayed fluorescence emitters. We introduce for the first time benzofuro[3,2-e]-1,2,4-triazine and benzothieno[3,2-e]-1,2,4-triazine acceptors which were connected to several common donors: phenoxazine, phenothiazine, carbazole and 3,6-di-tert-butylcarbazole. DFT calculations, and steady-state and time-resolved photophysical studies were conducted in solution and in solid states. While derivatives with azine moieties are non-emissive in any form, the compounds comprising 3,6-di-tert-butylcarbazole display TADF in all cases. More interestingly, the two derivatives substituted with a carbazole donor are TADF active when dispersed in a polymer matrix and phosphorescent at room temperature in neat films (microcrystalline form).


 Please wait while we load your content...
Please wait while we load your content...