Liquid ethylene glycol: prediction of physical properties, conformer population and interfacial enrichment with a refined non-polarizable force field†
Abstract
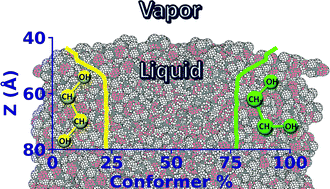
Periodic density functional theory based molecular dynamics simulations confirm the fraction of molecules in neat liquid ethylene glycol with their central OCCO dihedral in the trans conformation to be 21% at ambient conditions, while the rest are gauche conformers. Using this result as a benchmark, two non-polarizable force fields are developed herein to reproduce the conformer populations in the liquid, an important aspect inadequately addressed in several generic force fields. The mean dipole moment of a molecule in the liquid is estimated to be about 40% enhanced over its value in the gas phase, a feature discerned via AIMD simulations and fairly reproduced by our force fields. They are also shown to quantitatively predict all the physical properties of the liquid. Molecules present at the liquid–vapor interface of ethylene glycol are oriented with their methylene groups pointing towards the vapor phase, a requirement that enriches the interface with gauche conformers, in line with polarized sum frequency generation spectroscopy results.


 Please wait while we load your content...
Please wait while we load your content...