Adsorption of NO, NO2 and H2O in divalent cation faujasite type zeolites: a density functional theory screening approach
Abstract
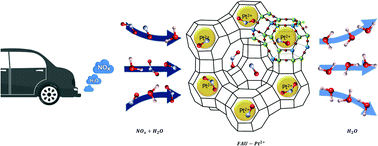
Emissions of diesel exhaust gas in confined work environments are a major health and safety concern, because of exposition to nitrogen oxides (NOx). Removal of these pollutants from exhaust gas calls for engineering of an optimum sorbent for the selective trapping of NO and NO2 in the presence of water. To this end, periodic density functional theory calculations along with a recent dispersion correction scheme, namely the Tkatchenko–Scheffler scheme coupled with iterative Hirshfeld partitioning TS/HI, were performed to investigate the interactions between NO, NO2, H2O and a series of divalent cation (Be2+, Mg2+, Ca2+, Sr2+, Ba2+, Fe2+, Cu2+, Zn2+, Pd2+, and Pt2+) faujasites. This enabled the identification of the optimum zeolites to selectively capture NOx in the presence of H2O, with respect to two important criteria, such as thermodynamic affinity and regeneration. Our results revealed that Pt2+ and Pd2+ containing faujasites are the best candidates for effective capture of both NO and NO2 molecules, which paves the way towards the use of these sorbents to address this challenging application.


 Please wait while we load your content...
Please wait while we load your content...