Understanding of the interactions between azole-anion-based ionic liquids and 2-methyl-3-butyn-2-ol from the experimental perspective: the cage effect†
Abstract
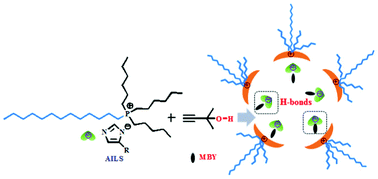
The interactions between azole-anion-based ionic liquids (AILs) and 2-methyl-3-butyn-2-ol (MBY) play an important role in AIL-promoted carboxylative cyclization of MBY with CO2. To better understand the interactions between AILs ([P66614][Im], [P66614][4-MeIm], and [P66614][4-BrIm]) and MBY, a detailed investigation from the experimental perspective has been carried out in this study. The results show that the derivative of viscosity (η) with the mole fraction of AIL (xAIL) of AIL + MBY mixtures appears to have the maximum value when xAIL ≈ 0.3, while 1H NMR chemical shifts of P-CH2 of [P66614]+ reach the minimum value at xAIL ≈ 0.3, indicating that [P66614]+ of AILs tend to self-aggregate. The interaction parameters (gji–gii) of the systems obtained from η by the Eyring-UNIQUAC equation are positive, and the difference between the bulk and local composition (xi–xii) is always negative, indicating that AILs can interact with MBY. Moreover, excess molar volumes and isentropic compressibility deviations are all negative deviations and become more negative as the temperature increases, reaching a minimum value at xAIL ≈ 0.30, indicating that azole-based anions can form H-bonds with MBY, and MBY molecules tend to enter the aggregates formed by AILs. Consequently, the cage effect is proposed to describe the interactions between AILs and MBY: MBY first enters the cage formed by the aggregation of [P66614]+, and then forms H-bonds with azole-based anions. Finally, the sizes of the particles of the [P66614][Im] + MBY mixture from dynamic light scattering increase first and then decrease with xAIL, with the maximum of 122 nm at xAIL ≈ 0.25, which confirms the rationality of the cage effect.


 Please wait while we load your content...
Please wait while we load your content...